Fetal Triple test
Fetal Triple test is a modern and innovative screening tool for the first trimester of pregnancy. It combines the benefits of two non-invasive tests: a fetal cell-free DNA chromosomal screening and carrier screening for both parents. Additionally, it incorporates three advanced ultrasound examinations conducted at various stages of fetal development.
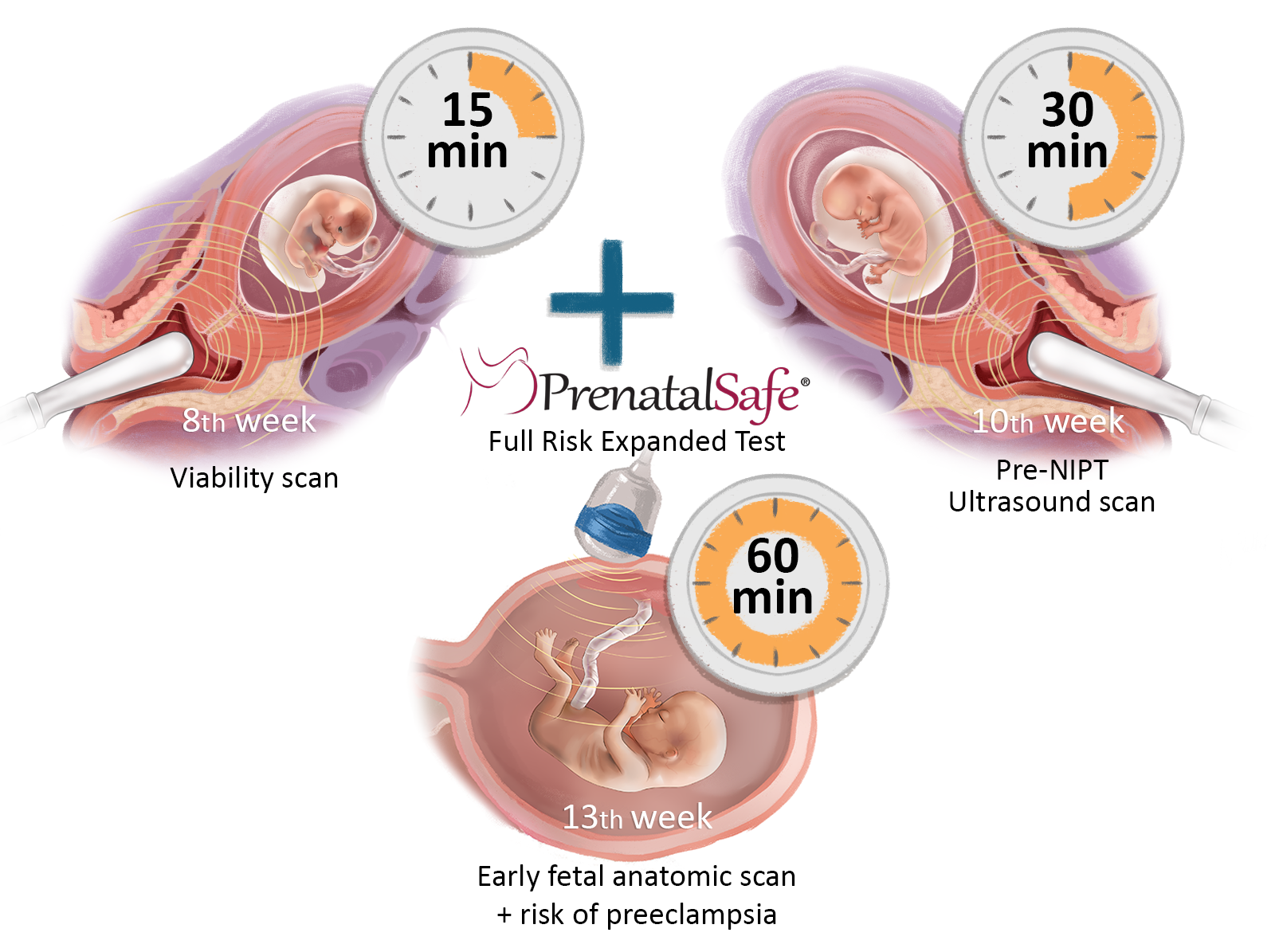
The Fetal Triple test is a comprehensive prenatal screening that includes several key examinations. At 8 weeks of pregnancy, it involves a fetal viability ultrasound along with the GeneScreen Complete test. At 10 weeks, a very early ultrasound examination for fetal malformations is conducted along with the KaryoPlus and GeneSafe Complete tests. Additionally, at 13 weeks, this process is combined with detailed fetal ultrasound diagnostics and maternal risk assessment. Together, these tests provide one of the most accurate methods for early detection of chromosomal and genetic diseases, as well as congenital malformations in children.
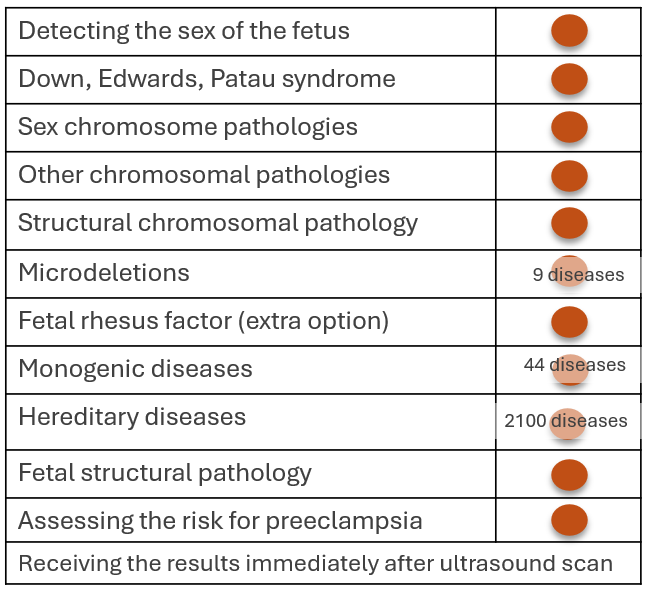
This combined screening test detects nearly 2,100 severe chromosomal or genetic diseases and congenital malformations in the fetus early, which would otherwise remain undetected prenatally or be discovered later in pregnancy.
-
The Fetal Triple test can detect the diseases listed below:
- Chromosome 22 aneuploidies (including Down syndrome, Edwards syndrome, and Patau syndrome);
- Sex chromosome anomalies (including Turner syndrome, Klinefelter syndrome, and Jacobs syndrome);
- Rare chromosome deletions and duplications <7Mb (including Williams syndrome);
- 9 clinically significant microdeletions (including DiGeorge syndrome);
- 44 different genetic diseases (including Noonan syndrome, Rett syndrome);
- 2100 hereditary monogenic diseases (including cystic fibrosis, genetic sensorineural deafness, spinal muscular atrophy, Tay-Sachs disease, Gaucher disease and phenylketonuria, Duchenne muscular atrophy, fragile X syndrome, Fabry disease, X-linked mental retardation, X-linked hydrocephalus);
- Major severe congenital malformations (including spina bifida);
- Major severe heart defects (including transposition of the great arteries).
In addition to detecting the pathology of the fetus, the Fetal Triple test test also provides an opportunity to find those women who may develop the following serious complications during pregnancy:
- Preeclampsia, in which the woman's kidney and liver function is disrupted, protein enters the urine and develops high blood pressure, which in more severe cases may be accompanied by a life-threatening seizure syndrome.
- Intrauterine growth retardation of the fetus, where the child does not reach its potential growth and lags in growth behind its peers. Due to a lack of food and oxygen, the gynecologist must monitor the growth and well-being of these fetuses with ultrasound more often and, if necessary, induce childbirth prematurely if the child's condition worsens.
- Premature birth due to a change in the structure of the cervix, as a result of which the cervix opens unnoticed during pregnancy, and the baby is born prematurely, when it is not yet able to cope with breathing outside the womb, maintaining the temperature and sucking on its own.
The Fetal Triple test is the best choice for parents who want to obtain as much information as possible about pregnancy risks and their unborn child using non-invasive testing methods.
-
The Fetal Triple test consists of three stages:
Step ONE: Fetal viability ultrasound and blood test for both mother and father for the GeneScreen Complete test at 8 weeks of pregnancy.
Step TWO: At the 10th week of pregnancy, we conduct a pre-NIPT ultrasound with pre-test counselling and blood analysis for the PrenatalSafe Complete Plus test (which includes the PrenatalSafe Karyo Plus test and the GeneSafe Complete test), along with blood test to determine placental hormones. During this stage, we also review the pregnancy history and measure arterial blood pressure, weight, and length. In addition to the mother's venous blood analysis, the test also requires an analysis of the child's father's oral mucosa.
Step THREE: At the 13th week of pregnancy, we perform an early diagnostic ultrasound examination of the fetus to check for developmental defects. After this examination, we will explain the results of the Fetal Triple test, answer any questions, and, if necessary, provide recommendations for the pregnancy management plan. It is important to note that if the GeneSafe test or GeneScreen test requires additional in-depth analysis by the laboratory, the results may take an additional 7–10 days beyond the initial timeline. In such cases, the GeneSafe or GeneScreen test results will not be available on the day of the ultrasound examination. Instead, they will be sent securely to the patient's email as soon as they are received from the laboratory, along with the doctor's explanations.
- Combined screening is available for individuals whose pregnancy has reached at least 8 full weeks (8 weeks + 0 days). To determine the duration of your pregnancy, please use our pregnancy calculator.
- The combined test also allows for the option to learn the sex of the fetus, if desired.
- However, please note that we only offer the Fetal Triple test for singleton pregnancies.
- Results for the PrenatalSafe Complete Plus test will be provided within 21 working days, while results for the GeneScreen Complete test will be available within 35 working days. If the GeneScreen Complete blood test is performed at the 8th week of pregnancy, followed by the PrenatalSafe Complete blood test at the 10th week, the woman will receive the written results of the combined screening, along with explanations, immediately after the ultrasound examination conducted at the 13th week of pregnancy.
It is essential to highlight the following points:
- The Fetal Triple test is not a substitute for the diagnostic ultrasound examination for fetal malformations conducted at the 20-week mark of pregnancy. The first-trimester ultrasound cannot identify all potential malformations.
- As a non-invasive screening test, the Fetal Triple test cannot detect all chromosomal and genetic disorders that can be identified through diagnostic amniocentesis performed at the 16-week stage of pregnancy.