The Fetal Ultrasound Center offers a new state of the art first trimester combined screening test - the Fetal Triple test.
Rare Disease Day is celebrated globally in February, coinciding with the rarest day of the year, February 29. A rare disease is defined as one that affects fewer than one in every 2,000 people. Over 8,000 rare diseases have been documented worldwide, but unfortunately, only 5% have an effective treatment available. The prevalence of these diseases affects approximately 6-8% of the population. While each rare disease is characterized by low incidence, the overall number of individuals affected by rare diseases is significant. In Estonia alone, around 100,000 people may be living with various rare diseases of differing severity.
Dr. Marek Šois, a gynaecologist, has collaborated with the Genoma Eurofins genetics laboratory in Rome to develop a new prenatal test for rare diseases called the Fetal Triple test. This test employs an innovative approach that combines the strengths of two different non-invasive prenatal tests (NIPT) based on fetal cell-free DNA for detecting hereditary diseases in both parents, along with advanced ultrasound examinations conducted at various stages of fetal development.
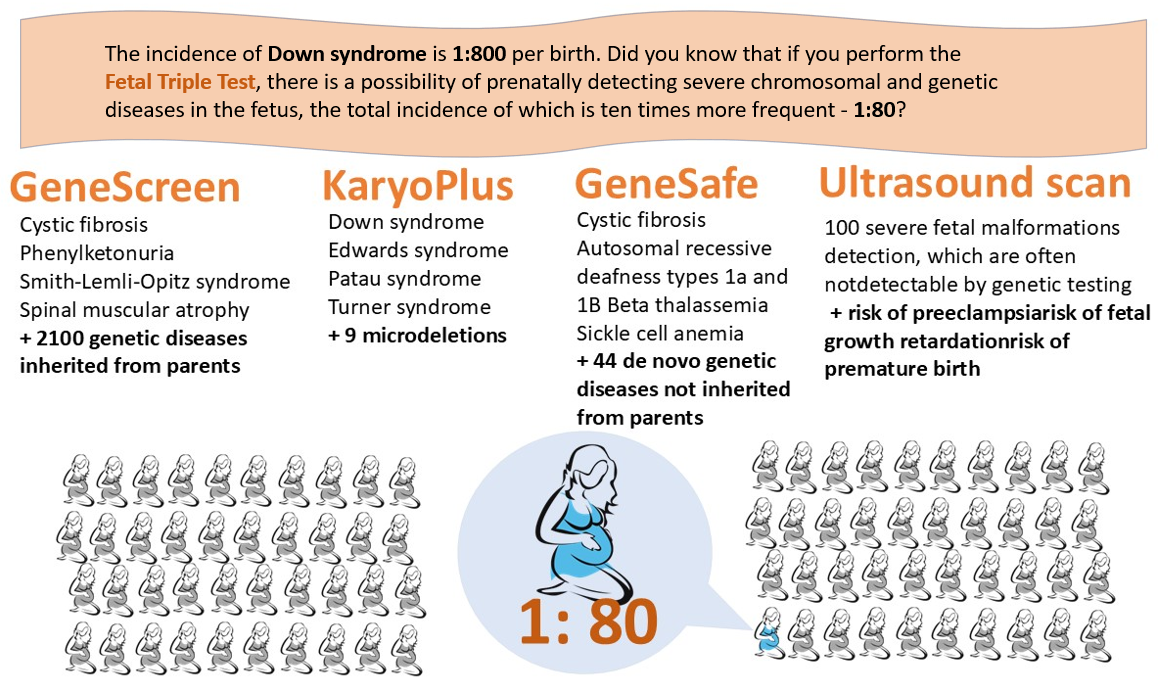
The Fetal Triple test can detect conditions in the fetus with an incidence of 1 in 80, which is ten times more common than the incidence of Down syndrome (1 in 800).
Currently, 1.4% (or 1 in 70) of newborns are born with some form of chromosomal or genetic disorder. Many children with rare diseases experience psychomotor, mental, or social developmental delays, often more severe than those seen in children with Down syndrome. After completing the Fetal Triple test, the residual risk of having a chromosomal or genetic disease is reduced to just 0.1% (or 1 in 1000).
The Fetal Triple test consists of the following separate studies:
Ultrasound Examinations. Three ultrasound examinations are conducted at different stages of the child's development:
- Pregnancy Detection and Fetal Viability Assessment.
This ultrasound examination is designed to assess fetal viability, rule out ectopic pregnancy, determine the size of the pregnancy, and evaluate the presence and type of multiple pregnancies. It can exclude five congenital malformations that are not compatible with life.
- Early Fetal Malformation Examination.
This examination aims to assess fetal viability and identify situations where the Non-Invasive Prenatal Testing (NIPT) should not be performed. It can exclude ten congenital malformations that are not compatible with life.
- Early Detailed Fetal Ultrasound Examination with Early Echocardiography.
This examination can detect up to 100 different congenital malformations that are often not identifiable through genetic tests. Nearly 80% of rare diseases have a genetic origin, meaning that one in five children with a rare disease would go undetected prenatally without the use of ultrasound. Additionally, this ultrasound measures blood flow indices in the woman’s uterine arteries and the length of her cervix, helping to assess the risk of preeclampsia, intrauterine growth restriction, and premature birth during the pregnancy.
Non-Invasive Chromosome and Genetic Disease Tests. Three non-invasive tests focus on chromosome and gene diseases:
- GeneScreen Complete Test.
As a screening tool for assessing genetic disease carrier status, this test evaluates the carrier status of 2,100 familial inherited diseases for both the woman and the father of the future child. If both parents are carriers of the same genetic disease, their child may inherit that disease.
- PrenatalSafe Karyo Plus Test.
This test detects numerical and structural chromosomal abnormalities and identifies nine clinically significant microdeletions from the mother's venous blood, in addition to Down, Edwards, and Patau syndromes, as well as sex chromosome pathologies that can significantly affect the child's health.
- GeneSafe Complete Test.
This test provides information on 29 different fetal single-gene pathologies, which can result in 44 distinct newly developed genetic diseases that are not inherited from the parents. It also identifies five genetic diseases inherited from the parents, which are frequently not detected prenatally by ultrasound and may only manifest later in pregnancy or in early childhood.
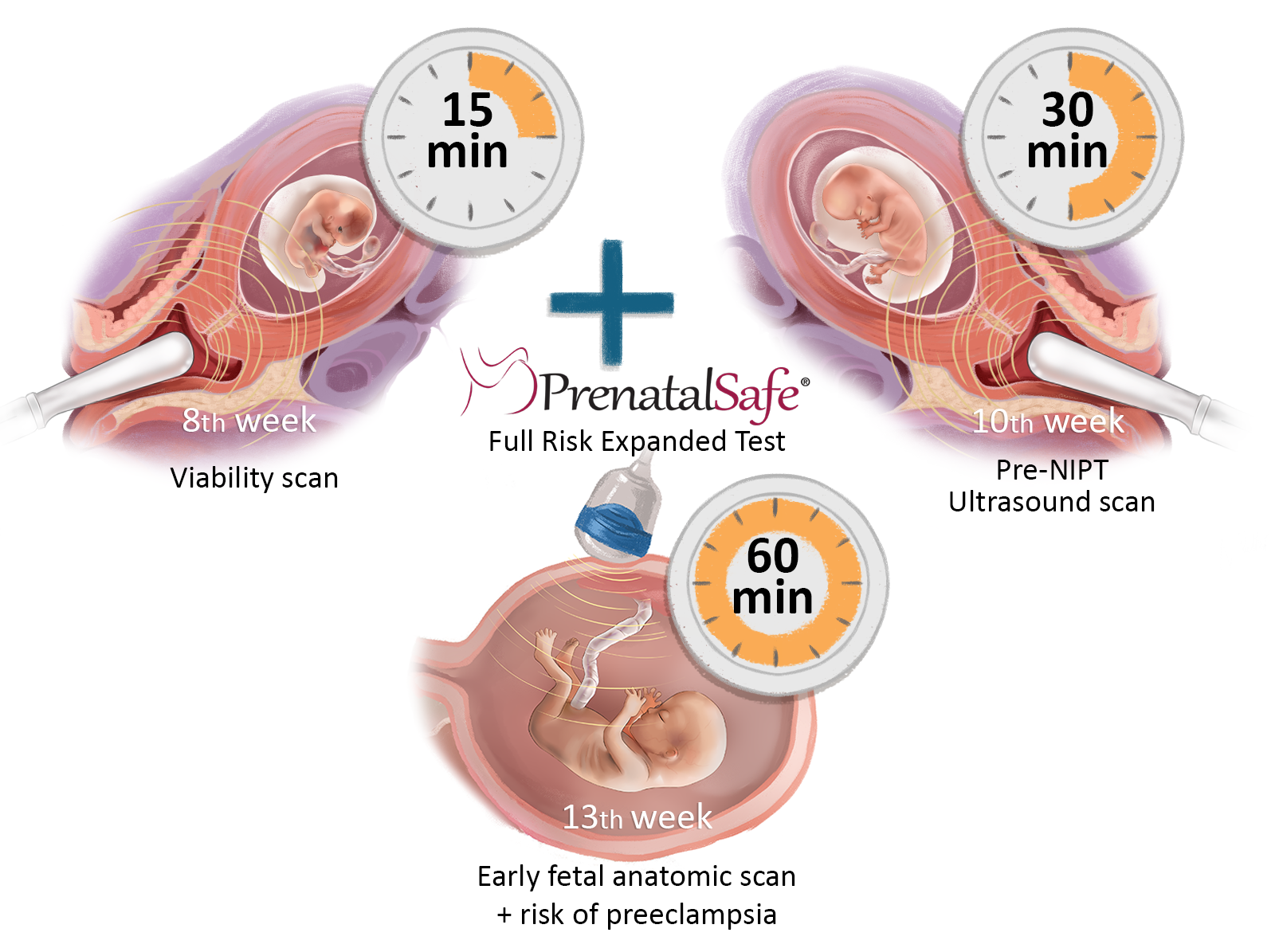
The Fetal Triple test consists of three stages:
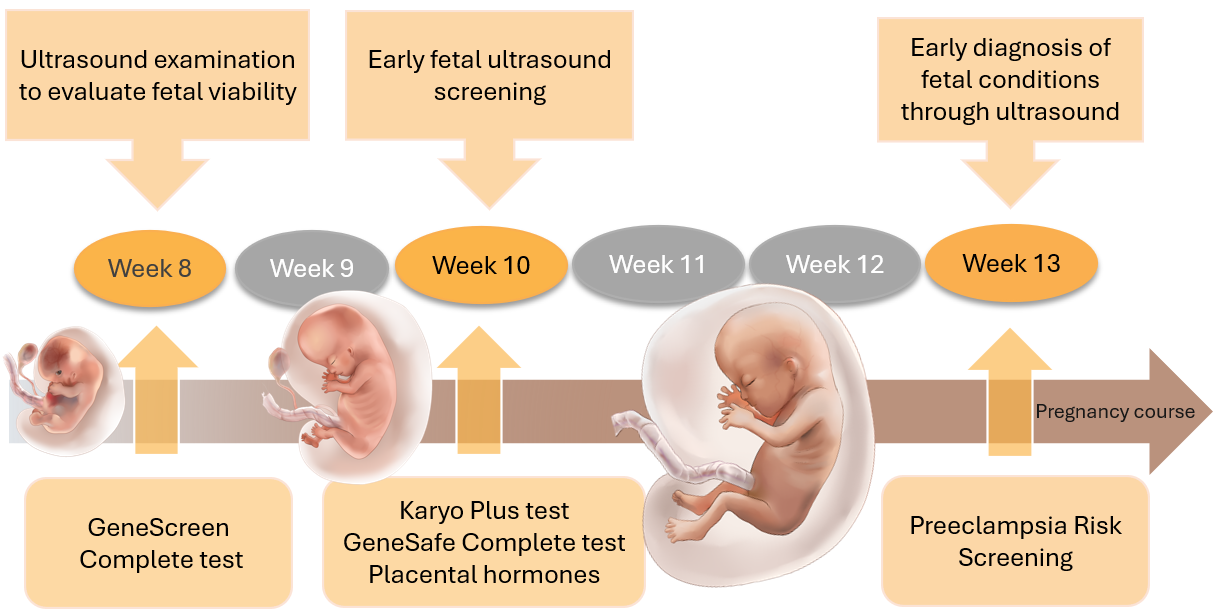
- Step ONE: Fetal viability ultrasound and blood test for both mother and father for the GeneScreen Complete test at 8 weeks of pregnancy.
- Step TWO: At the 10th week of pregnancy, we conduct a pre-NIPT ultrasound and blood analysis for the PrenatalSafe Complete Plus test (which includes the PrenatalSafe Karyo Plus test and the GeneSafe Complete test), along with blood test to determine placental hormones. During this stage, we also review the pregnancy history and measure arterial blood pressure, weight, and length. In addition to the mother's venous blood analysis, the test also requires an analysis of the child's father's oral mucosa.
- Step THREE: At the 13th week of pregnancy, we perform an early diagnostic ultrasound examination of the fetus to check for developmental defects. After this examination, we will explain the results of the Fetal Triple NIPT test, answer any questions, and, if necessary, provide recommendations for the pregnancy management plan.
The Fetal Triple test is a combined screening test that can detect nearly 2,100 severe chromosomal and genetic diseases, as well as congenital developmental defects in the fetus at an early stage. Many of these conditions would remain undetected until later in pregnancy or might go unnoticed altogether. Additionally, the Fetal Triple NIPT test can determine the sex of the fetus if desired.
The Fetal Triple test can identify the following health issues:
- Chromosome 22 aneuploidies (including Down syndrome, Edwards syndrome, and Patau syndrome);
- Sex chromosome anomalies (including Turner syndrome, Klinefelter syndrome, and Jacobs syndrome);
- Rare chromosome deletions and duplications less than 7 Mb (including Williams syndrome);
- Nine clinically significant microdeletions (including DiGeorge syndrome);
- 44 different genetic diseases inherited from parents (including Noonan syndrome, Rett syndrome, and Usher syndrome);
- 2,100 genetic diseases inherited from parents (including cystic fibrosis, genetic sensorineural deafness, spinal muscular atrophy, Tay-Sachs disease, Gaucher disease, phenylketonuria, Duchenne muscular dystrophy, fragile X syndrome, Fabry disease, X-linked mental retardation, and X-linked hydrocephalus);
- Major severe congenital malformations (including spina bifida);
- Major severe heart defects (including transposition of the great arteries).
In addition to detecting fetal pathologies, the Fetal Triple test can also help identify women at risk for serious complications during pregnancy, such as:
- Preeclampsia: A condition where the woman's kidneys and liver function are impaired, leading to protein in the urine and high blood pressure. In severe cases, this can result in life-threatening seizures.
- Intrauterine growth restriction: When the fetus does not reach its growth potential and falls behind its peers. Due to inadequate nutrition and oxygen, healthcare providers need to monitor these fetuses more closely with ultrasounds and may need to induce labor early if the child's condition worsens.
- Premature labor: Caused by changes in the structure of the cervix, leading to premature opening without the woman's awareness. This can result in the child being born prematurely, which can pose serious health risks.
The Fetal Triple test is an excellent choice for parents who wish to gain comprehensive knowledge about the risks associated with pregnancy and their unborn child through a combination of non-invasive testing methods.
By participating in the Fetal Triple test, a woman can gain confidence in her child's health as well as the overall health of the pregnancy.